Taking a Look at How Aluminum Is Made
 Aluminum is a very useful form of metal. It is one of
the most abundant of them on earth. However, it is not typically found in
pure form. Aluminum exists most commonly as a part of a compound with one or
more different elements. It must be either recycled from prior use or
refined from other elements to which it is bound.
Aluminum is a very useful form of metal. It is one of
the most abundant of them on earth. However, it is not typically found in
pure form. Aluminum exists most commonly as a part of a compound with one or
more different elements. It must be either recycled from prior use or
refined from other elements to which it is bound. Aluminum is appreciated for being a strong, yet easily malleable metal for industrial application. This makes it a resilient material that can be easily recycled once its use expires.
Historically, aluminum was put to use by ancient cultures in compound form. However, it was not until nearly the 20th century that it was isolated as a specific element which can be used to form a unique metal.
The most common material from which aluminum can be produced is Bauxite. Aluminum is extracted from bauxite ore in a two part process. The Bayer and Hall-Heroult procedures are used in succession to separate pure aluminum from the other elements in bauxite.
Bauxite was originally discovered by Pierre Berthier in Les Baux, a French town after which it is named. It is gathered via strip mining. Since it is found so close to the top layer of earth, it is not a complicated mining procedure. However, it can have some adverse environmental impact; vegetation must be cleared away. This can harm the natural ecosystem upon which wildlife relies. This is one reason why environmentally-conscious consumers choose to recycle aluminum.
Bauxite ore is combined with lye(sodium hydroxide), a strong base, which catalyzes a chemical reaction. Aluminum oxide, also known as alumina, is extracted from the bauxite compound. This is called the Bayer process.
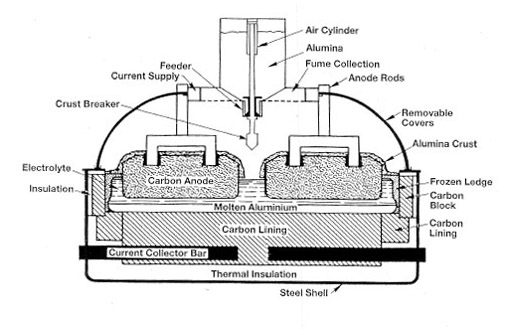
Once the alumina is created, the Hall-Heroult process is used to create pure aluminum. Alumina is mixed with cryolite. This combination undergoes electrolysis. At this stage, the liquid aluminum can be separated from carbon dioxide.
Aluminum can also be generated via a recycling process. This process can differ based on how the aluminum was originally used. For example, aluminum wheels have wheel bearings made of other materials; these must be physically removed. Generally, once the aluminum is isolated from foreign materials, it is crushed into bales. It is heated to allow the aluminum to separate from other materials that might have bound to it. The aluminum is melted into liquid form and refined into the preferred storage shape. Industrial metals are often sold as ingots; this is true for aluminum as well.
Both means can produce workable aluminum of good quality. Recycling is a very common way to create aluminum; it is so commonly used in commercial goods that there are great quantities to employ in the easy recycling process. Since waste aluminum would likely be discarded anyways, many consumers choose to take their scrap aluminum to recycling. They are often rewarded financially for such a decision, motivating a continuous supply of relatively pure aluminum to return to the industrial process.
Although it is very abundant, refining aluminum can be quite complicated. It was only made available in pure form for industrial use fairly recently; prior to the late 1800s it could only be used in compounds with other elements. Luckily, the recycling process allows us to recirculate refined aluminum without too much effort.
Copyright © 2012 scrapaluminumprices.net
